Abstract
As the cornerstone electrolyte solute for lithium-ion batteries, Lithium Hexafluorophosphate (LiPF6) directly dictates cycle life and thermal safety. This paper provides a deep dive into the kinetic mechanisms of the LiPF6 + H2O reaction and the destructive impact of its byproducts on the internal cell interfaces. By quantifying the LiPF6 decomposition pathways, we establish benchmarks for high-purity electrolyte selection and environmental control standards for global battery manufacturers.
I. Physico-chemical Essence and Industry Landscape
In high-energy density systems, LiPF6 maintains over 90% market share due to its superior ionic conductivity and electrochemical window. Global search trends indicate that technical interest is highly concentrated in South Korea, Japan, and the USA—regions at the forefront of battery innovation.
1.1 Precision Physical Parameters
- Density & Morphology: A white crystalline powder with a standard density of 1.50.
- Thermal Instability: LiPF6 is inherently thermo-sensitive. Decomposition initiates at 60°C, accelerating significantly at higher temperatures; by 180°C, the decomposition rate can reach 50%. This necessitates rigorous thermal management systems (BMS) in end-use applications.
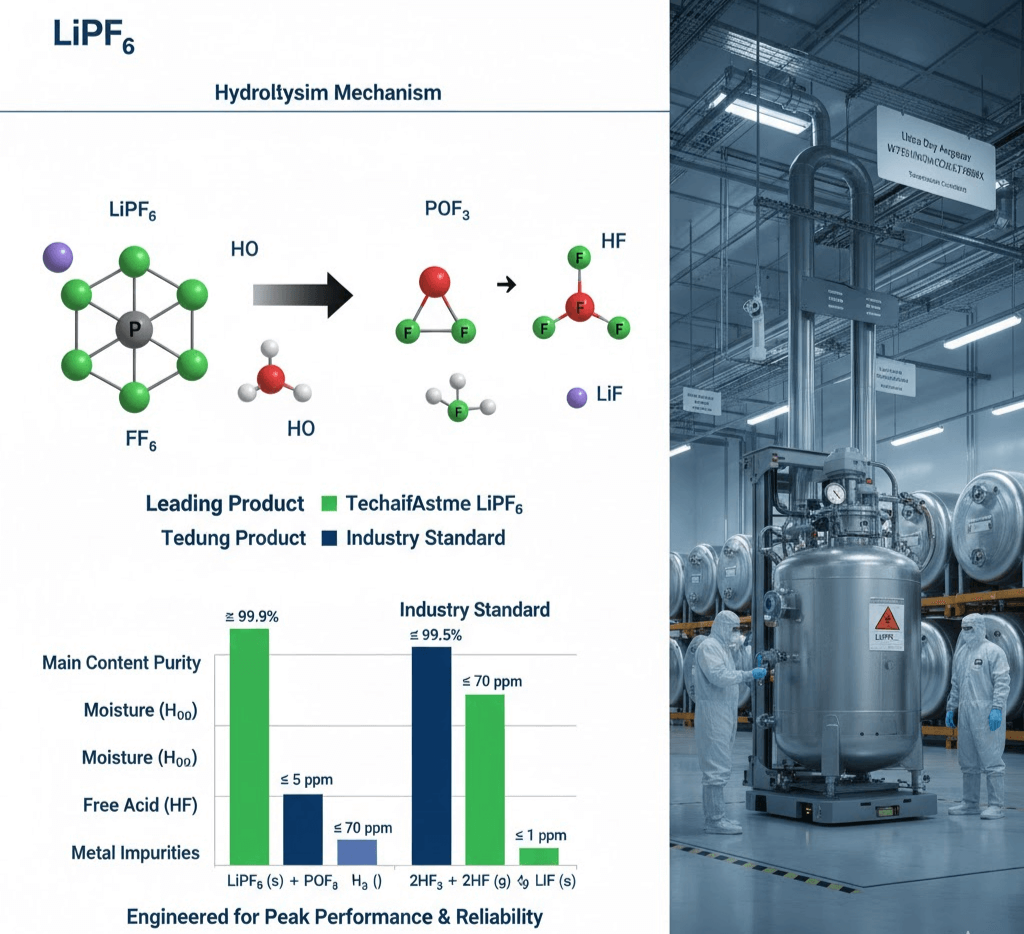
II. Core Mechanism: Deep Dive into the LiPF6 + H2O Hydrolysis Chain
The extreme moisture sensitivity of LiPF6 is regarded as the “Quality Red Line” in industrial production.
2.1 Hydrolysis Kinetics
When ambient or solvent moisture levels exceed the critical threshold of 10 ppm, LiPF6 undergoes an immediate and irreversible exothermic decomposition:
LiPF6+ H2O → POF3 ↑ + 2HF + LiF ↓
2.2 The “Systemic Failure” Caused by Byproducts
- Hydrofluoric Acid (HF) Erosion: The generated HF is a potent inorganic acid that attacks the lattice of cathode active materials (e.g., NCM/LFP), leading to transition metal dissolution and migration.
- SEI Layer Degradation: HF continuously consumes the Solid Electrolyte Interphase (SEI) on the anode, causing a spike in interfacial resistance (Rsei) and rapid capacity fade.
- Insoluble Precipitates: Produced LiF crystals deposit within electrode pores, hindering lithium-ion desolvation and transport kinetics.
III. Expert-Level Procurement Benchmarks: PPM-Grade Quality Standards
When evaluating the LiPF6 price, technical decision-makers must look beyond unit cost to these ultra-high-spec technical indicators to mitigate latent failure costs:
| Key Specifications | Leading Industry Standard | Technical Insight |
| Main Content | ≥ 99.9% | Ensures maximum theoretical ionic conductivity. |
| Moisture (H2O) | ≤ 5 ppm | Significantly below the 10 ppm decomposition threshold. |
| Free Acid (as HF) | ≤ 70 ppm | Minimizes initial acidification and extends cell cycle life. |
| Insolubles | ≤ 150 ppm | Prevents filter clogging and micro-short circuits. |
| Metal Impurities | Each ≤ 1 ppm | Minimizes self-discharge rates (Fe, Na, K, Ca, etc.). |
IV. Global Supply Chain Safety and Compliance (SDS & SOP)
Due to its hazardous nature, logistics and storage must adhere to stringent Safety Data Sheet (SDS) protocols:
- Atmospheric Control: Must be stored in sealed containers pressurized with high-purity inert gas (Argon or Nitrogen).
- Thermal Management: Store in cool, shaded, and locked areas, strictly isolated from heat sources.
- Incompatibility: Keep away from oxidants and moisture; storage areas must be designated as “Water-Prohibited” (No automatic sprinklers).
V. Conclusion: Long-term Value in Chemical Stability
Understanding the LiPF6 + H2O reaction is the fundamental bedrock for pushing the limits of energy density and cycle performance. For global B2B procurement, selecting a product with 99.9% purity and <5 ppm moisture is a strategic move to eliminate systemic risks such as battery swelling and sudden capacity drop at the source.







